Which Transition Emits Light With The Shortest Wavelength?

How Do I Know If Someone Restricted Me On Instagram, 3 Ways To Know If Someone Muted You On Instagram (2022), 3.34 MB, 02:26, 48,002, How To Apps, 2021-08-13T18:32:09.000000Z, 19, Can find someone on Instagram that you have blocked if they have, www.quora.com, 602 x 1070, png, blocked deleted, 20, how-do-i-know-if-someone-restricted-me-on-instagram, KAMPION
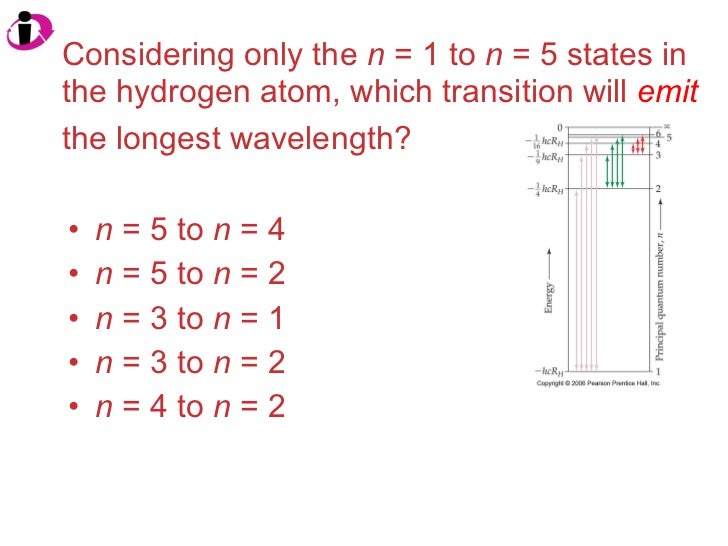
Which one of the transitions would produce a visible blue light? Determine the allowed orbit in this atom. The transition that emits light with the shortest wavelength is n = 5 to n = 1. The energy difference among the given options is the greatest from n = 5 to n = 1 which means it will have the shortest wavelength.
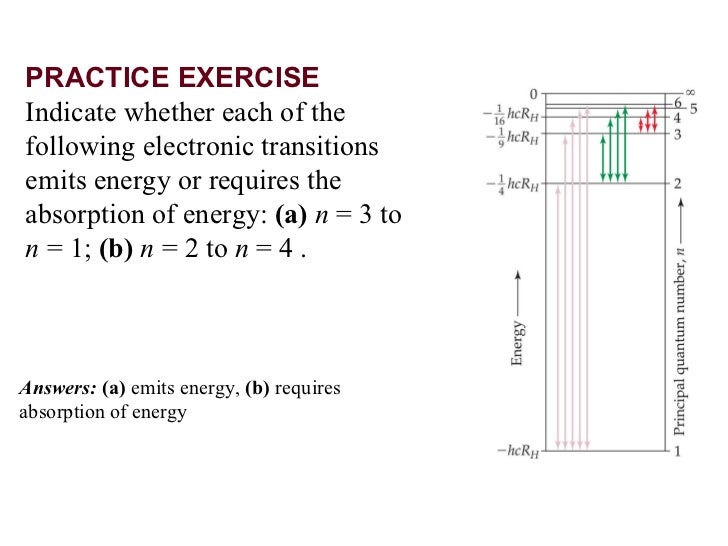
#c =# the speed of light. We can rearrange this formula to get. This shows that the wavelength is inversely proportional to the energy: The smaller the amount of energy absorbed, the longer the wavelength. So, we look for the transition that involves the smallest energy. The electron transitions between first and fourth energy levels (n 4 → n 1 ) in the hydrogen atom produces the line of shortest wavelength in hydrogen spectrum. Among the given transitions, the energy difference for the transition n 4 → n 1 is maximum. Higher is the energy difference, lower is the wavelength. The transition that gives light the shortest wavelength is the one that produces the most energy change.
M8-S7: Bohr's and Rutherford's Atomic Models and their Limitations

Chapter 6 Lecture- Electrons in Atoms
Answered: 9. Which transition in the hydrogen… | bartleby

Which electron jump in a hydrogen atom absorbs the photon of highest

Solved: Which Of The Following Transitions In A Hydrogen A... | Chegg.com

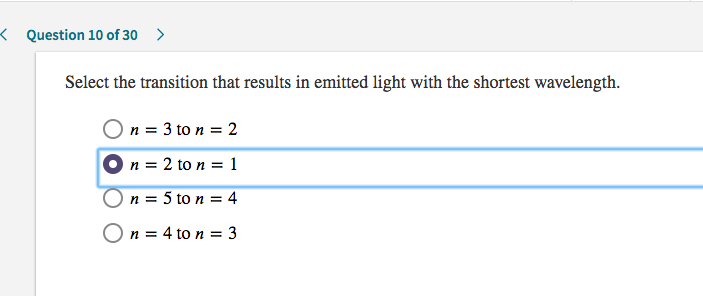
Solved: KQuestion 10 Of30 > Select The Transition That Res... | Chegg.com
Hydrogen Atom: Hydrogen Atom Longest Wavelength
Hydrogen atom absorbs radiations of wavelength `lambda_0` and

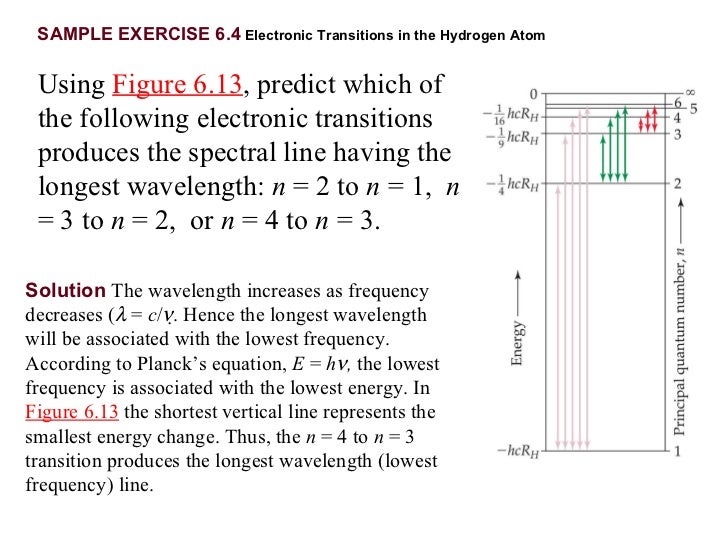
PPT - What is the wavelength of the longest wavelength light that can
Chapter 6 Lecture- Electrons in Atoms
Komentar
Posting Komentar